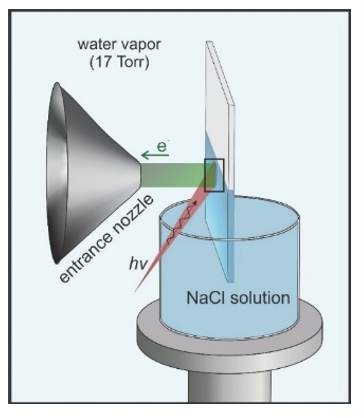
Scientific Achievement
Using tender-APXPS, we have directly measured the Donnan potential at the membrane/solution interface for CR-61 membranes equilibrated with NaCl and MgCl2 solutions.
Image: A schematic of the experimental setup during data acquisition from the CR-61 membrane equilibrated in aqueous salt solution.
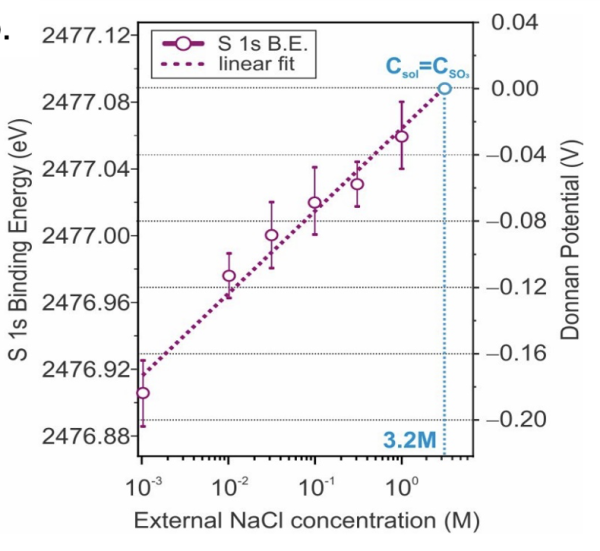
Significance and Impact
This study demonstrates the first direct measurement of the Donnan potential, which classic textbooks claim cannot be measured. This discovery expands our understanding of the ion partitioning in membranes and has the potential to impact a wide range of scientific fields, including energy, water purification, and biological sciences.
Image: Experimental measurements of the Donnan potential from spectral binding energy shifts of sulfonate group related core as a function of external solution concentration.
Research Details
APXPS and “dip and pull method” was used to measure the binding energy shifts in the core level peaks of the membrane as a function of the external salt concentration and shown to be directly related to the Donnan potential.
We have compared our experimentally determined potentials with values predicted using well-established thermodynamic models, providing the first test of applicability of these models in estimating the Donnan potential.
Notes
Selective transport of charged solutes across the membrane is a critical step for many electrochemical, biological, and colloidal systems. For biological systems, the selective ion transport is achieved through highly specialized ion channels. Similarly, ion exchange membrane (IEM) based technologies, which are built on membrane permselectivity between counter- and co-ions, play a critical role in meeting increasing global demands for energy and water. In 1911, permselectivity of charged membranes was postulated for the first time by Prof. Frederick G. Donnan, regarding the formation of an electrical potential at the membrane/solution interface. The so-called Donnan potential was found to be responsible for the selective transport of ions. Although the literature on Donnan equilibrium spans many decades, it is still widely reported that the Donnan potential cannot be measured directly.
In this work, for the first time, we report the direct probing of the Donnan potential of an IEM equilibrated with aqueous salt solutions using tender (hv = 4 keV) ambient pressure X-ray photoelectron spectroscopy (APXPS), a technique capable of probing chemical and electrostatic information of buried solid/liquid interfaces. Our ability to directly measure the Donnan Potential of IEM is novel and exciting, as it has been thought to be unmeasurable and has never been accomplished in the over 100 years since the original Donnan theory was proposed. Our results directly reveal the dependence of the membrane’s Donnan potential on external salt concentration and counter-ion valence, as suggested by Donnan himself. In addition, we have compared our experimentally determined potentials with values predicted using well-established thermodynamic models, providing the first test of applicability of these models in estimating the Donnan potential.
Acknowledgements
This work was supported as part of the Center for Materials for Water and Energy Systems (M-WET), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award # DE-SC0019272. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility, under contract no. DE-AC02-05CH11231. J.Q., Y.W., and E.J.C. were partially supported by an Early Career Award in the Condensed Phase and Interfacial Molecular Science Program, in the Chemical Sciences Geosciences and Biosciences Division of the Office of Basic Energy Sciences of the U.S. Department of Energy, under Contract No. DE-AC02-05CH11231.
Aydogan Gokturk, et al., Nature Comm., accepted

