Scientific Achievement
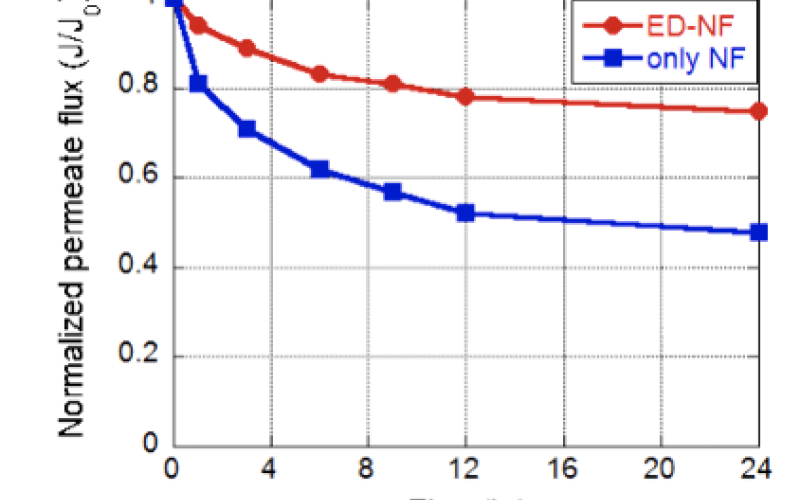
Selective removal of divalent cations (e.g., Ca+2) via electrodialysis prior to nanofiltration reduces membrane fouling, which reduces permeate flux decline and improves boron rejection.
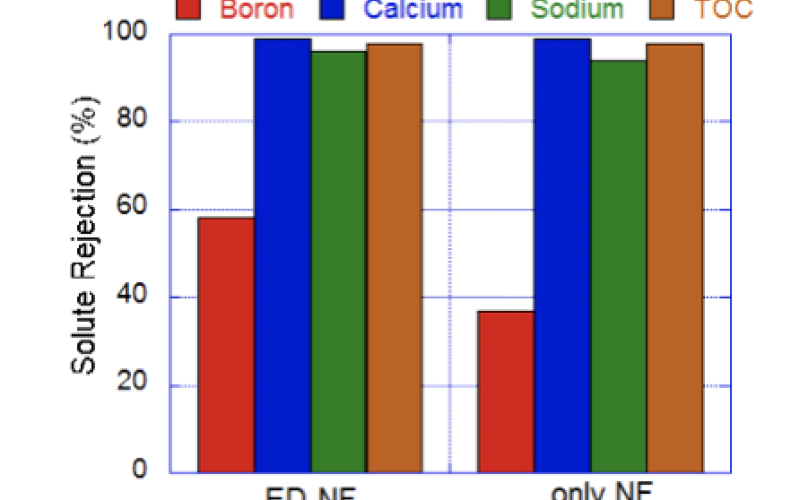
Image: ED pretreatment increases boron rejection by NF due to reductions in fouling/scaling
Significance and Impact
The interactions that cause membrane fouling can be minimized through selective pretreatment, thus optimizing the performance of membrane systems.
Research Details
- Electrodialysis removes charged ions (e.g. Ca2+, CO32-), but neutral species (e.g. B(OH)3 at circumneutral pH) are not separated.
- Following ED pretreatment, pH of a complex water can be increased to create negatively charged borate ion species without CaCO3 precipitation or fouling.
- Fouling reductions achieved with ED pretreatment increase solute-membrane interactions that drive boron rejection.
The Integrating Framework is investigating the use of electrodialysis (ED) as a pretreatment option for nanofiltration (NF) as a means of reducing membrane fouling and improving the removal of challenging solutes such as boron. Boron is difficult to remove in conventional membrane systems due to its small size and neutral speciation at circumneutral pH. Current practices often employ pH adjustments prior to filtration to improve boron removal. However, in complex water systems, pH adjustments lead to membrane fouling and scaling, including calcium-carboxylic interactions and calcium carbonate precipitation.
The selective removal of calcium and other ions by ED reduces these inorganic-organic interactions and enables pH adjustments without scaling concerns. ED experiments were completed using water with 3% salinity containing: calcium, sodium, boron, and alginic acid. The results shown underscore the benefits of incorporating ED at circumneutral pH before increasing the pH for NF. These benefits include reductions in permeate flux declines due to calcium-carboxylic interactions and increased boron rejection. We theorize that the increased boron rejection demonstrated may be due to reductions in concentration polarization as well as increased electrostatic repulsions between the membrane surface and charged boron. Current work is addressing the mechanisms of action to guide the development of novel membrane materials for neutral solutes.
Work was performed at The University of Texas at Austin.

