Scientific Achievement
Reverse LiCl/NaCl selectivity was attained by incorporating 12-crown-4 into a membrane.
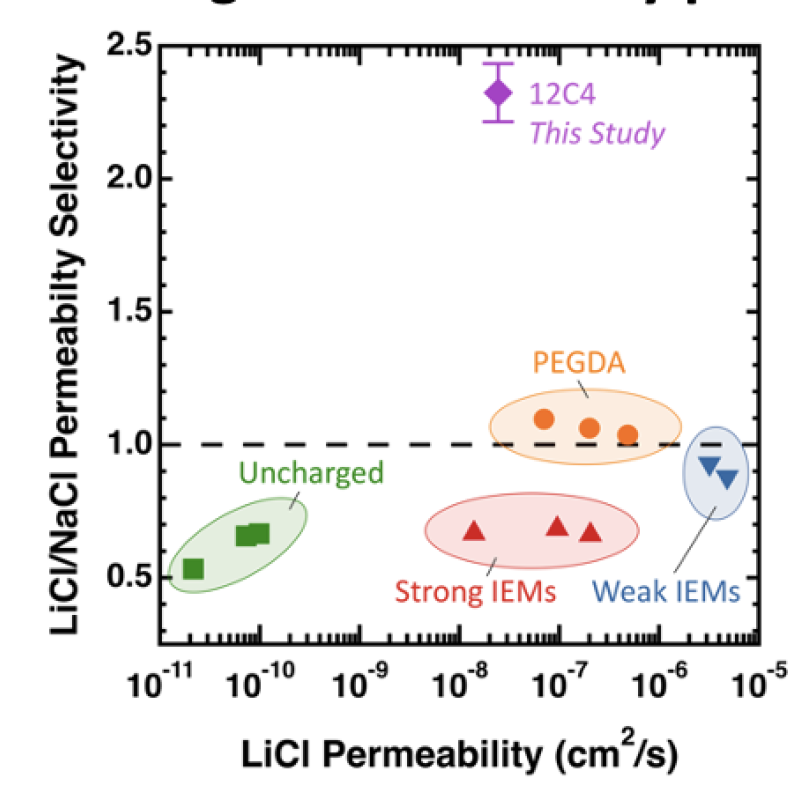
Image: 12-crown-4 membranes exhibit unprecedented reverse LiCl/NaCl selectivity.
Significance and Impact
Provided insights into how host-guest chemistry influences ion transport/selectivity in polymer membranes, which is critical to the design of Li+ recovery processes.
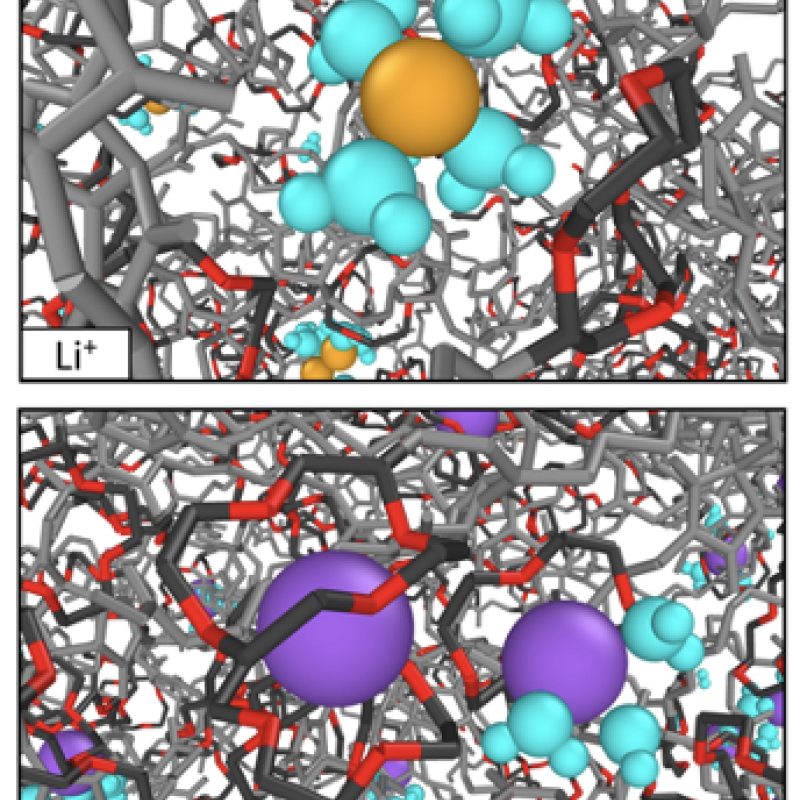
Image: Simulations revealed that this selectivity is due to stronger interactions between 12-crown-4 and Na+ (12-crown-4 = black and red, Li+ = yellow, Na+ = purple, H2O = blue).
Research Details
- Developed a polymer platform based on ring opening metathesis polymerization of functionalized norbornene monomers
- Macroscopic transport measurements revealed unusual reverse LiCl/NaCl permeability selectivity based on diffusivity
- Molecular dynamics simulations elucidated the molecular basis for selectivity: Based on hydration free energy, Na+ complexes strongly with 12-crown-4, significantly reducing its mobility, while Li+ shows little interaction with 12-crown-4 due to limited dehydration
Lithium is widely used in contemporary energy applications, but its isolation from natural reserves is plagued by time-consuming processes. While polymer membranes could circumvent these challenges by efficiently extracting lithium from aqueous solutions, they usually exhibit poor ion-specific selectivity. Toward this end, we’ve incorporated host-guest interactions into a tunable polynorbornene network by copolymerizing: (1) 12-crown-4 ligands to impart ion selectivity, (2) poly(ethylene oxide) side-chains to control water content, and (3) a crosslinker to form robust solids at room temperature. Single salt transport measurements indicate these materials exhibit unprecedented reverse permeability selectivity (~2.3) for LiCl over NaCl—the highest documented to date for a dense, water-swollen polymer.
As demonstrated by molecular dynamics simulations, this behavior originates from the ability of 12-crown-4 to bind Na+ ions more strongly than Li+ in an aqueous environment, which reduces Na+ mobility (relative to Li+) and offsets the increase in Na+ solubility due to binding with crown ethers. Under mixed salt conditions, 12-crown-4 functionalized membranes showed identical solubility selectivity relative to single salt conditions; however, the permeability and diffusivity selectivity of LiCl over NaCl decreased, presumably due to flux coupling. These results reveal new insights for designing advanced membranes with solute-specific selectivity by utilizing host-guest interactions.
SJ Warnock, R Sujanani, ES Zofchak, S Zhao, TJ Dilenschneider, KG Hanson, S Mukherjee, V Ganesan, BD Freeman, MM Abu-Omar, CM Bates (In Press).
Work was performed at University of California Santa Barbara and The University of Texas at Austin.

