Scientific Achievement
Elucidated the mechanism of lithium selectivity in 12-Crown-4-functionalized membranes.
Significance and Impact
Provided molecular level insights into how free volume and ion complexation impact monovalent ion selectivity in ligand functionalized membranes.
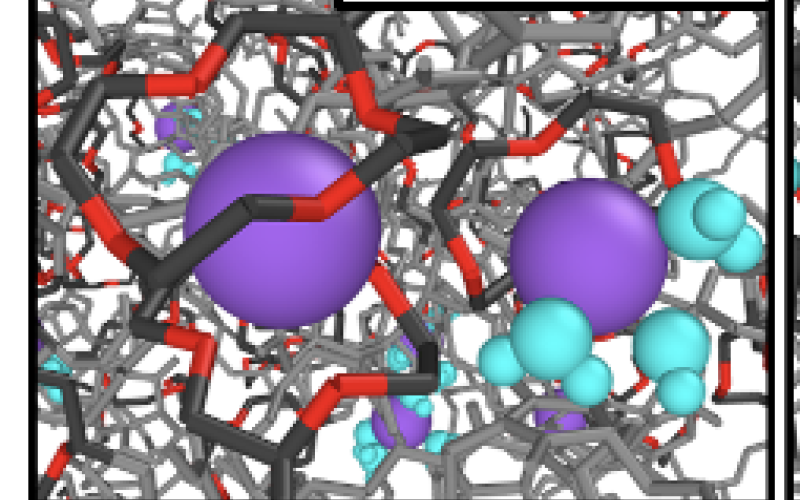
NaCl Membrane
(Image 3)
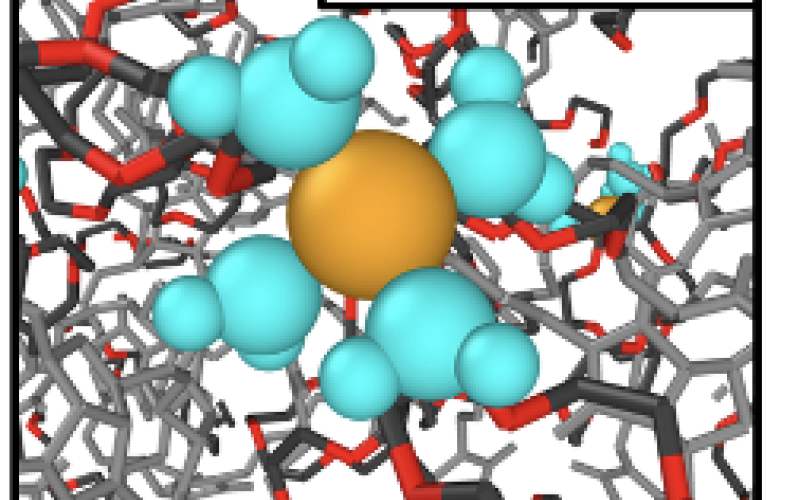
NaCl Membrane
(Image 4)
Research Details
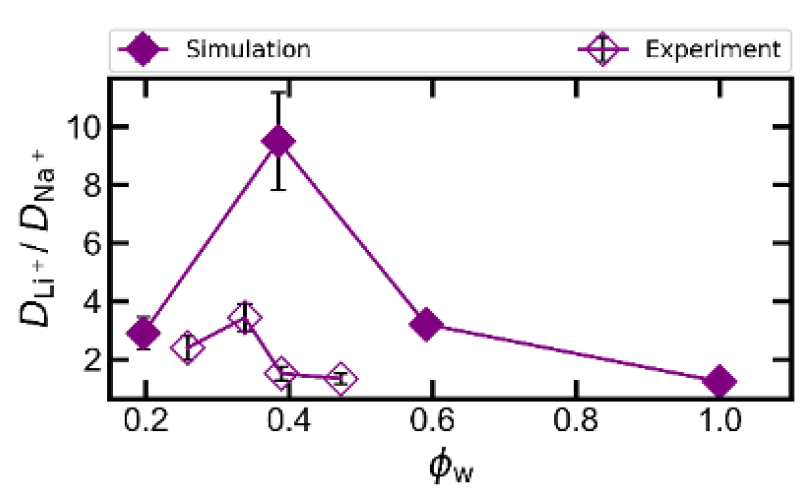
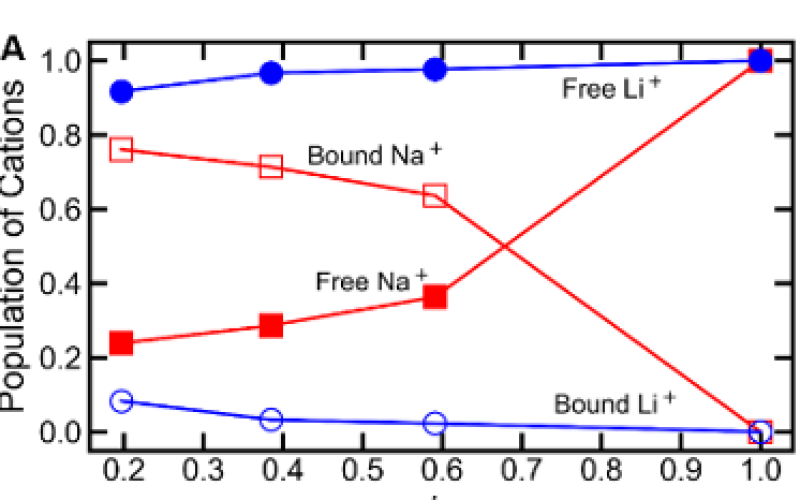
- Li/Na selectivity arises from selective complexation of Na by 12-Crown-4, reducing Na diffusivity relative to Li.
- Na complexation decreases with increasing water volume fraction, offsetting the increase in Li selectivity due to an increase in the size and dispersity of free volume elements.
- Ion diffusivity in the membrane can be modeled as the free ion fraction multiplied by free ion diffusivity, opening potential avenues for predicting diffusivity selectivity in ligand-functionalized membranes.
Direct lithium extraction via membrane separations has been fundamentally limited by the lack of monovalent ion selectivity exhibited by conventional polymeric membranes, particularly between sodium and lithium ions. Recently, a 12-crown-4-functionalized polynorbornene membrane was shown to have the largest lithium/sodium permeability selectivity observed in a fully aqueous system to date. Using atomistic molecular dynamics simulations, we reveal that this selectivity is due to strong interactions between sodium ions and 12-crown-4 moieties which reduce sodium ion diffusivity while leaving lithium ion mobility relatively unaffected.
Simulation results suggest that a non-monotonic trend in lithium/sodium diffusivity selectivity with increasing water volume fraction results from a competition between an increasing free volume size and dispersity (increasing selectivity) and a decreasing population of bound sodium (decreasing selectivity). Ion diffusivities in the membrane, when scaled by their respective solution diffusivities and free ion fractions, are observed to collapse on to an almost universal relationship depending on solvent volume fraction. These results have implications for future ligand-grafted membrane design and provide physical insight into the origins of diffusivity selectivity in these materials.
ES Zofchak, Z Zhang, BK Wheatle, R Sujanani, SJ Warnock, TJ Dilenschneider, KG Hanson, S Zhao, S Mukherjee, MM Abu-Omar, CM Bates, BD Freeman, V Ganesan (Submitted).
Work was performed at The University of Texas at Austin and University of California Santa Barbara.

