Scientific Achievement
Fundamental ion transport properties in membranes synthesized with either the same charge density or the same water uptake were characterized. These properties were compared to established models to build structure-property relationships.
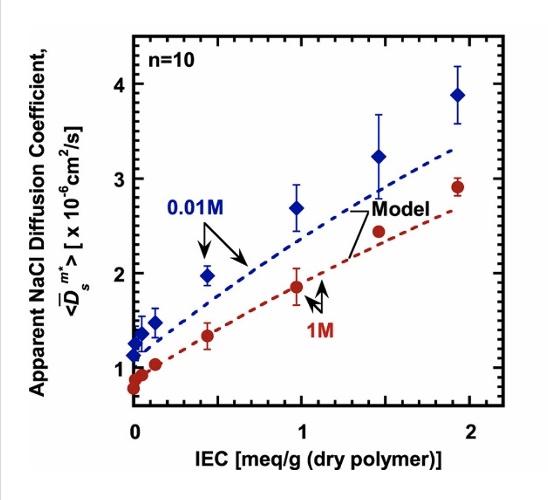
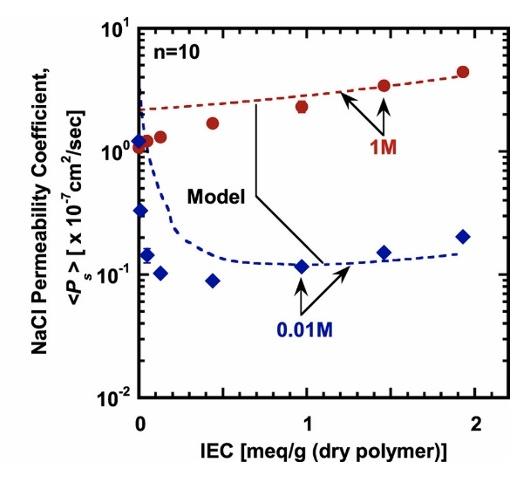
Image: NaCl diffusion (left) and permeability (below) coefficients as a function of membrane IEC values (meq of charge/g dry polymer). The dashed lines denote model predictions based on the Mackie and Meares ion diffusion model and the Donnan/Manning ion sorption model.
Significance and Impact
Decoupling the impact of water and charge content on ion transport in membranes is often difficult. By preparing and studying a systematic series of hydrogel membranes, the generality and limitations of current theoretical models was investigated.
Image: NaCl diffusion (above) and permeability (left) coefficients as a function of membrane IEC values (meq of charge/g dry polymer). The dashed lines denote model predictions based on the Mackie and Meares ion diffusion model and the Donnan/Manning ion sorption model.
Research Details
Hydrogel membranes were prepared using UV irradiation
Salt permeability, water permeability and ionic conductivity were measured
Theory and experiment were in good agreement for ionic conductivity in highly charged membranes.
Notes
This manuscript reported salt and ion transport properties in synthesized polymers exhibiting controlled charge density and water uptake. Given their ion selective nature, charged polymers are important for water purification and energy generation applications. Salt and ion transport properties are central to membrane performance and are frequently correlated to charge density and water uptake. However, systematic studies connecting polymer structure and ion transport properties in charged polymers are not widely available. Through careful adjustment of synthesis conditions, polymers with varied charge and water content were prepared. To develop structure-property relationships, salt and ion transport properties were experimentally measured and interpreted using the solution-diffusion model, the Nernst-Planck equation, the Donnan/Manning model, and the Mackie and Meares model. By utilizing a system with broadly varied structural properties, these results highlighted the generalities and limitations in current theories.
Acknowledgements
This work was supported as part of the Center for Materials for Water and Energy Systems (M-WET), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award #DE-SC0019272.
Editor choice article
https://twitter.com/J_Membrane_Sci/status/1537706613745778692
Related Publication
Ni Yan, Rahul Sujanani, Jovan Kamcev, Eui-Soung Jang, Kentaro Kobayashi, Donald R. Paul, and Benny D. Freeman, "Salt and Ion Transport in a Series of Crosslinked AMPS/PEGDA Hydrogel Membranes," Journal of Membrane Science, 653, 120549, 2022 July 5, https://doi.org/10.1016/j.memsci.2022.120549.

