Scientific Achievement
We show that partitioning dominates over diffusivity to dictate ideal salt permeation in ligand-functionalized polymer membranes.
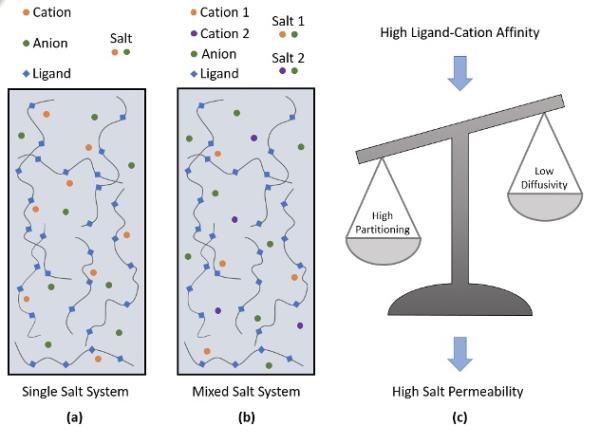
Image: Schematic description of (a) single and (b) mixed salt systems. (c) partitioning dominates over diffusivity to dictate ideal salt permeation in ligand-functionalized polymer membranes.
Significance and Impact
Ideal salt permeation is seen to increase with increase in cation-ligand interaction strength in both single and mixed salt systems. This knowledge will be helpful in designing novel polymer membranes with improved ion selectivity.
Research Details
- Molecular Dynamics and Grand Canonical Monte Carlo simulations were conducted to probe ideal salt diffusivity and salt partitioning into the membrane, respectively.
- Parametric study was conducted by varying the cation-ligand interaction strength. Both single and mixed salt systems were probed by altering the mixture composition.
Notes
Ion−ion separation is critical to several industries such as pharmaceuticals, biotechnology, refineries, and water purification. Recently, ligand-functionalized polymer membranes have started receiving attention as a scalable and cheap alternative for carrying out ionic separation processes. The ligands exhibit different binding affinity to the various ions, thereby creating an inherent asymmetry in the ionic transport process. In general, the ions having stronger affinity to the ligands partition more into the ligand-functionalized polymer membrane. However, this increase in partitioning is counteracted by a decrease in ionic diffusivity. Thus, there is an inherent tradeoff between the partitioning and diffusivity of the underlying species which ultimately dictates the permselectivity of the membrane.
Our team at The University of Texas at Austin conducted coarse-grained molecular dynamics simulations to probe the impact of cation-ligand interaction strength on ideal salt transport in ligand-functionalized polymer membranes. Our results reveal that the increase in salt partitioning dominates over the decrease in ideal salt diffusivity within the membrane, thereby enhancing the ideal salt permeability as cation-ligand interaction strength increases. In addition to probing single salt systems, we also investigate the transport of binary salt mixtures sharing a common anion, commonly known as mixed salt systems. We reveal that the mixed salt systems exhibit much more selective partitioning of salts into the membrane as compared to their single salt counterparts. However, the single salt systems are seen to exhibit higher ratio of ideal salt diffusivities than their mixed salt analogs. Fascinatingly, we observe that mixed salt systems always exhibit higher ideal permselectivity than single salt systems when the ligand-selective cation has a relatively high affinity towards the ligands.
Acknowledgements
This work was supported as part of the Center for Materials for Water and Energy Systems (M-WET), an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under Award no. DESC0019272. We also acknowledge support from Welch Foundation (F-1599). The results in this paper were generated using high-performance computing resources provided by The University of Texas at Austin Texas Advanced Computing Center.
Simulations were conducted using computational resources provided by the University of Texas Advanced Computing Center.
Related Publication
Harbor Singh Sachar, Everett S. Zofchak, Nico Marioni, Zidan Zhang, Sanket Kadulkar, Tyler J. Duncan, Benny D. Freeman, and Venkat Ganesan, "Impact of Cation-Ligand Interactions on the Permselectivity of Ligand-Functionalized Polymer Membranes in Single and Mixed Salt Systems, Macromolecules, 55(11), 4821-4831, 2022 May 17, https://doi.org/10.1021/acs.macromol.2c00543.

