Scientific Achievement
Molecular simulations show that longstanding measures and conventional thinking about solute hydrophobicity fail to capture affinity (binding) to different surfaces.
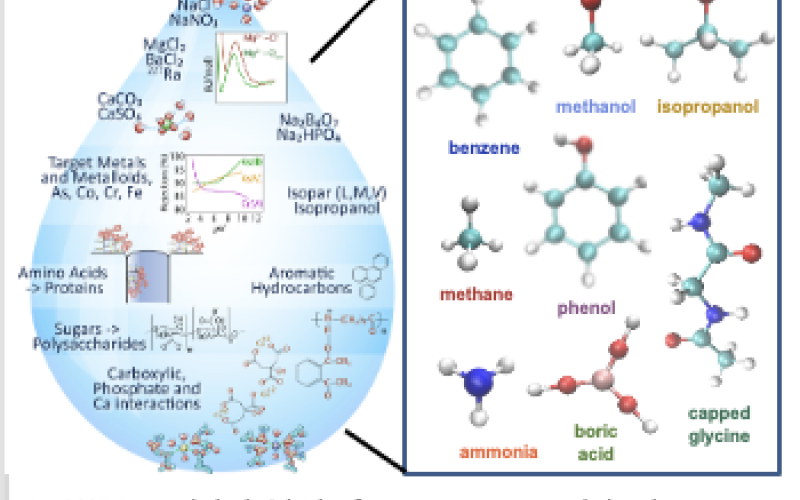
Image: M-WET Model Fluid Platform - For a variety of representative small solutes in produced water, affinity to a hydrophobic surface is not well-correlated to solute solubility from the gas phase (Henry’s constant) nor to solute octanol-water partition coefficients or transfer free energies.
Significance and Impact
These results suggest new ways of predicting water-mediated surface adsorption through detailed analysis of hydration water structure, dynamics and properties.
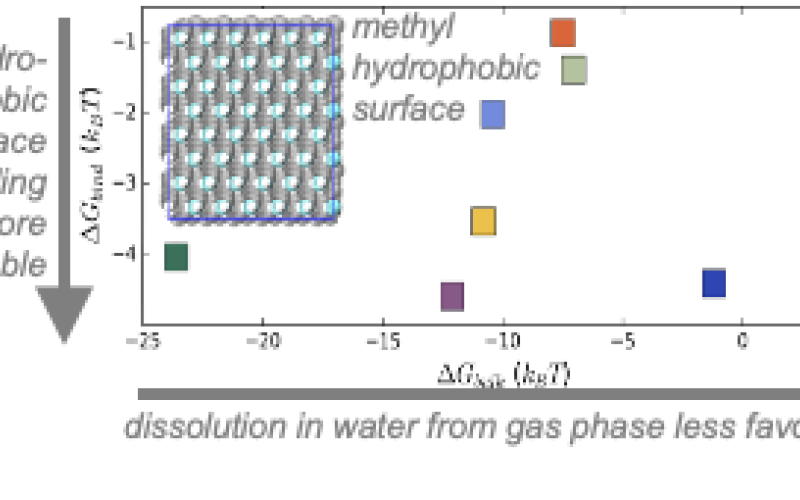
Image: M-WET Model Fluid Platform - Hydrophobic surface binding and dissolution in water from the gas phase.
Research Details
- Understanding the affinity of aqueous solutes to soft surfaces underpins rational design of fouling-resistant and energy efficient water purification membranes.
- Many studies focus on hydrophilicizing membrane surfaces to reduce fouling by hydrophobic solutes.
- Simulations showed that conventional measures of solute hydrophobicity fail to predict surface affinity, and that even hydrophilic surfaces attract hydrophobes. Hydration water structure explains these trends.
The operational performance of many water purification membranes is strongly influenced, at a fundamental level, by the structure, thermodynamics, and dynamics of interfacial water. Surface water plays a key role in particular for modulating solute adsorption and fouling, which significantly impairs performance. Much work has therefore focused on hydrophilizing membrane surfaces to reduce fouling, based on macroscopic assumptions and heuristics that hydrophilic interfaces are less subject to adsorption. However, a molecular understanding of solute-to-surface affinity is necessary for rational surface design rules that go beyond these current Edisonian approaches, for which molecular simulations provide a natural context. Here, simulations of a variety of solutes represented in produced water reveal that conventional whole-molecule characterization of solute hydrophobicity – through hydrophathy scales, solubility, or oil water-partitioning – fail to predict solute-surface affinities. Instead, context is important; specifically, the manner by which a given solute (or surface) perturbs hydration water structure provides more robust signatures of affinity.
Work was performed at the University of California - Santa Barbara.

