In this Project, we will gain understanding of ion hydration and its impact on ion association and provide insight for tuning membrane structures to control water and solute transport.
GAP B, Project 1: Role of Ion-ion and Ion-polymer Interactions on Ion Transport and Separations in Dry to Wet Systems
In this Project, we will gain understanding of ion hydration and its impact on ion association and provide insight for tuning membrane structures to control water and solute transport. Specifically, we will combine Clément’s pulsed field gradient (PFG)-NMR and novel electrophoretic NMR (eNMR) techniques with electrochemical and mass transport measurements (Freeman, Segalman) and mesoscopic simulations (Ganesan) under controlled hydration, connecting a new molecular level understanding of ion association and hydration with its impact on macroscopic transport in UMCP-derived materials using MFP solutes that are both NMR-active and relevant for water/energy applications (e.g., Li+, K+ Na+, Mg2+, Al3+, PO43-, F-, and TFSI- ions). The novel NMR tools, along with ion aggregation/dissociation analysis previously used by Clement and Segalman in dry polyelectrolyte systems enable the unique determination of ion self-diffusion coefficients and electrophoretic mobilities. These will be merged with macroscopic measures of ion association and ion diffusion typical in water purification systems (Freeman, Katz).
We propose synthesizing a homopolymer model system based on the porous UMCP in which we have tethered ligand groups (Fig. 3.3A). These functionalities range from bulky moieties with delocalized charges based on ionic-liquid chemistries to the denser charges more common in ion exchange membranes. Inclusion of dithiol functional groups allows us to very carefully control crosslink density and therefore the water uptake. Larger charged functional groups lead to weaker electrostatic interactions, but these can be overcome by modest thermal energies such that temperatures at or near room temperature provide substantial ion mobility in dry systems. At UCSB and UT, we have developed a detailed understanding of how segmental motion, ligand-bonding chemistry, backbone dielectric constant, and salt dissolution interplay in dry ion conduction in poly ionic-liquids (PILs). Further, Bates will develop specific interactants to impart ion-size and valency-based selectivity.
We hypothesize that the strength of ligand binding to the solute ions will be influenced by water content and will, in turn, alter both salt dissociation (ion aggregation/pairing) and the dynamics of ion motion. We will probe the role of solute anion and cation identity on the ionic conductivity and selectivity of these dry, decorated membranes in mono- and multivalent salts (LiCl, LiTFSI, NaCl, NaTFSI, KCl, KTFSI, MgCl2, BaCl2, AlCl3) at a range of ion concentrations. Building on the results for dry polymers, we will investigate the role that water plays in these properties by exposing samples to humid air in a quartz crystal microbalance and observing the resultant water uptake and changes in ionic conductivity. We will then, in the IF, correlate these observations with macroscopic changes in ion solubility, permeability, ionic conductivity, and selectivity as a function of water uptake (Katz, Kumar, Freeman). The investigation of alkali cations paired with organic fluorinated anions enables PFG-NMR and eNMR characterization, while also serving as an important bridge between ion dynamics in water filtration and energy focused systems.
Calculation of ion diffusion coefficients from ion permeability and ionic conductivity values using the Nernst-Planck relationship assumes a lack of ion aggregation/pairing. However, ion aggregation (referred to as pairing or clustering in different communities) can be prevalent in such systems and is anticipated to depend sensitively on the extent of membrane hydration, as well as membrane structure, morphology, and ion properties (charge, size, etc.). Yet, our quantitative understanding of the molecular mechanisms that govern ion sorption/transport in water purification membranes is limited to rather crude, continuum level models for highly charged and highly hydrated IEMs that assume complete dissociation, with no systematic connection to less highly charged or neutral polymers. Establishing this connection will require combining the above measurements with non-traditional approaches aimed at establishing a molecular level understanding of ion behavior.
We compare electrochemically measured conductivities, macroscopic ion sorption, and NMR based diffusion measurements to probe the extent of ion aggregation as a function of membrane chemistry and hydration levels. NMR chemical shift analysis of ions in solution will be used to quantify the extent of ion solvation with increasing membrane hydration. We will compliment this analysis with PFG-NMR measurements of anion (𝐷_), cation (𝐷+), and water (𝐷H2O) self diffusion coefficients and eNMR measurements of diffusion under a bias to garner insight into the relationship between membrane hydration and ion/water transport mechanisms. Furthermore, ion self-diffusion measurements will be used to calculate the Nernst-Einstein conductivity, which reflects the average diffusion of all paired and unpaired ions within the membrane (𝜎NE = (INSERT EQUATION) 𝑐i𝐷i, where 𝐹 is Faraday’s constant, R is the molar gas constant, and 𝑧i, 𝑐i, and 𝐷i are the charge, concentration, and ion diffusion coefficients). Together with electrochemical impedance measurements, which account for only charged ionic species, we will quantify the extent of ion aggregation in membranes via the ionicity: 𝐼 = (INSERT EQUATION), where 𝜎EIS. is the conductivity obtained via electrochemical means (cf. Fig. 3.4). Complementing such efforts, atomistic molecular dynamics simulations (Ganesan) will be used to elucidate the mechanistic underpinnings of the experimental observations. Explicitly, the framework of Onsager coefficients will be employed to identify the specific ionic correlations (for e.g., concerted movement of cations and anions) responsible for the ionicity. Such analyses will be combined with other measures such cluster size distribution to elucidate the origins of such dynamical correlations. Such simulations, performed for model metal ions and their counter-ions, will bridge theoretical descriptions of ion diffusion in wet and dry systems (Ganesan), while informing transport dynamics beyond the present macroscopic continuum models. Freeman will complement these efforts with macroscopic membrane measurements (i.e., permeability, ionic conductivity, solubility, diffusivity, and free volume) in the IF.
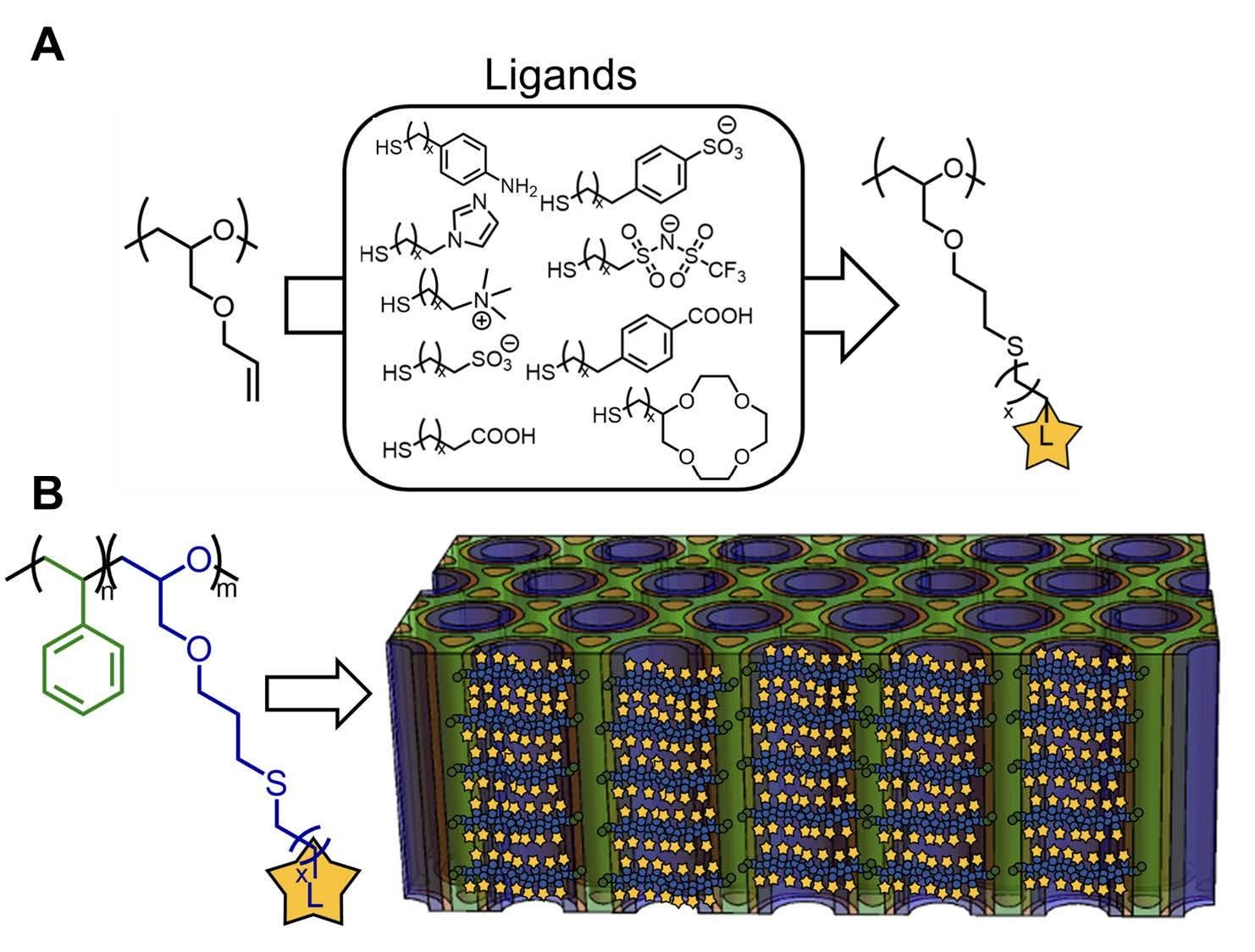
Fig. 3.3. The poly (allyl glycidyl ether) (PAGE) element of the porous UMCP offers a platform for study of multiple ligand (L) chemistries with the same polymer backbone. The polymers will be decorated by efficient Thio-Michael addition chemistry to yield materials with ligands on every monomer. Ligands of particular interest are amine, imidazole, sulfonic acid, carboxylic acid, and TFSI elements shown and will be studied in a homopolymer format in Project 1 (A). This same platform will be leveraged in a block copolymer format (B) such that the role of confinement can be studied in Project 2.
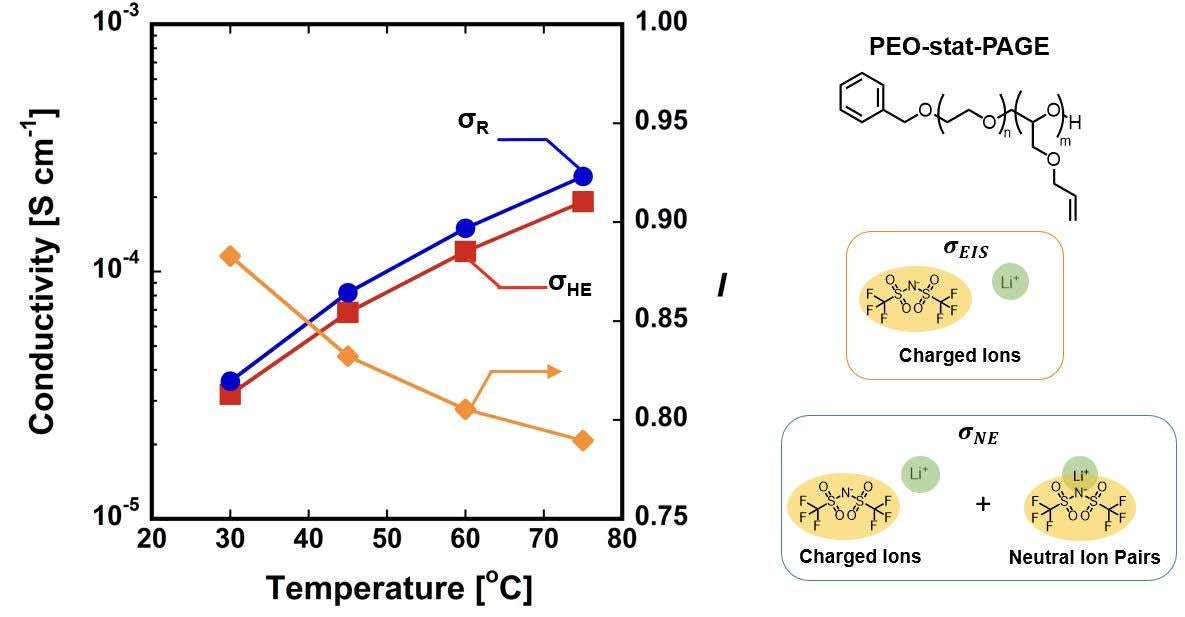
Fig. 3.4. Ion conductivity measured via electrochemical impedance spectroscopy (σEIS) is sensitive to only the presence of charged ions whereas diffusivities measured via PFG-NMR account for both charged ions and neutral ionic pairs/clusters and can be used to reconstruct a conductivity via the Nernst-Einstein equation (σNS). The ionicity, I, (i.e., (σEIS/σNS)) indicates the degree of ion pairing. Here, the ionicity decreases as a function of temperature indicating that ion pairing increases with increasing temperature. We anticipate hydration will have the opposite effect.

