The role of environmental parameters (e.g., confinement, solution composition, and degree of membrane hydration) on ion selectivity (or binding affinity) is often attributed to their impact on the media’s bulk dielectric constant.
GAP B, Project 2: Role of Local Polarity on Ion Association/Aggregarion and Water Interactions
The role of environmental parameters (e.g., confinement, solution composition, and degree of membrane hydration) on ion selectivity (or binding affinity) is often attributed to their impact on the media’s bulk dielectric constant. While continuum level electric field strength changes are incorporated directly into ion activity/sorption models using a dielectric constant (e.g., Manning’s model, Born solvation theory), bulk dielectric constants alone do not capture molecular scale effects on local water/functional group environments responsible for ion and proton binding. For example, some researchers have suggested that local environmental factors (e.g., water content) are responsible for changes in acid dissociation constants (pKa) in membrane networks. Others find no evidence for such changes, which points to missing fundamental science in this area. Using elements of the porous UMCP, we have a unique opportunity to create carefully controlled confined environments for understanding the role of local polarity on proton and ion affinity and the associated thermodynamic parameters that govern separation.
In Project 2 of GAP B, we will use an identical platform of functionalized UMCP elements as Project 1 (Gap B), but now in a self-assembling block copolymer (Fig. 3.3B) with a polystyrene matrix and functionalized/PEO-filled lamellae and cylindrical pores (5-40 nm). Study of solute and solvent transport in functionalized pore interiors will facilitate understanding of local polarity and electrostatic effects in confined systems. In the IF, Katz, Christopher, and Su will study local polarity effects on selectivity and permeability by introducing selected ions and water/alcohol mixtures. Then, using both synchrotron (X-ray absorption spectroscopy to interrogate ion-ligand interactions, and X-ray scattering to monitor changes in block copolymer pore structure) and macroscopic experiments (tritium exchange titrations, ion titrations, confocal microscopy) as well as bulk membrane characterization via the IF, proton and ion sorption and diffusion will be characterized. Varying the water/alcohol concentration provides an alternative approach (i.e., besides changing membrane chemistry) to systematically vary macroscopic dielectric constant, which has been shown to have a strong influence on pKa. In parallel with the above experimental measurements, Henkelman’s MD simulations will track ion dissociation and movement. The use of ML force fields based on DFT data will be key for describing fluctuations in local pKa/pH where variations in local charge environments are important. This approach will allow us to identify the importance of ion-ion interactions and hydration near these charged interfaces and will complement efforts in GAP A to understand the role of surface properties on ion affinities and water structure and dynamics.
Further, the local pH within porous materials containing selected ions and water/alcohol mixtures will be measured using confocal fluorescence microscopy. This approach has proven successful for rapid measurements of local pH in porous materials for applications ranging from corrosion to electrocatalysis. To measure local pH over broad ranges, we will use a combination of pH sensitive dye molecules (LysoSensor green DND-189 (LSG, pH = 1-4), 5(6)-carboxynaphthofluorescein (CNF, pH = 5-8.5), and a ratiometric two-color pH- sensitive fluorescent dye, 6,8-dihydroxy-1,3-pyrenedisulfonic acid (DHPDS, pH = 5-11.5). Measurements of local pH will enable correlations with continuum models and provide measured values for correlation with macroscopic measurements of transport and binding affinity.
The extent of protonation of ionizable groups in polymers can determine the effective fixed charge concentration of the polymer networks. At a given pH, the extent of protonation increases with ligand pKa, simultaneously increasing electrostatic repulsion of mobile ions from the polymer network and increasing water uptake, which enhances ion and water transport in swollen hydrogels (Fig. 3.2 and Fig. 3.5). We hypothesize that the introduction of readily protonated functional groups should increase permeability and selectivity simultaneously. The experiments described above will enable us to test this hypothesis. Moreover, because confinement plays a key role in surface functionalization, hard X-ray and soft/tender resonant X-ray scattering will be used to decipher changes in the pore size and spacing with enhanced contrast between blocks, leveraging recent developments by Su to characterize morphology of hydrated membranes with soft and tender X-rays and correlating these changes to changes in pKa and affinity.
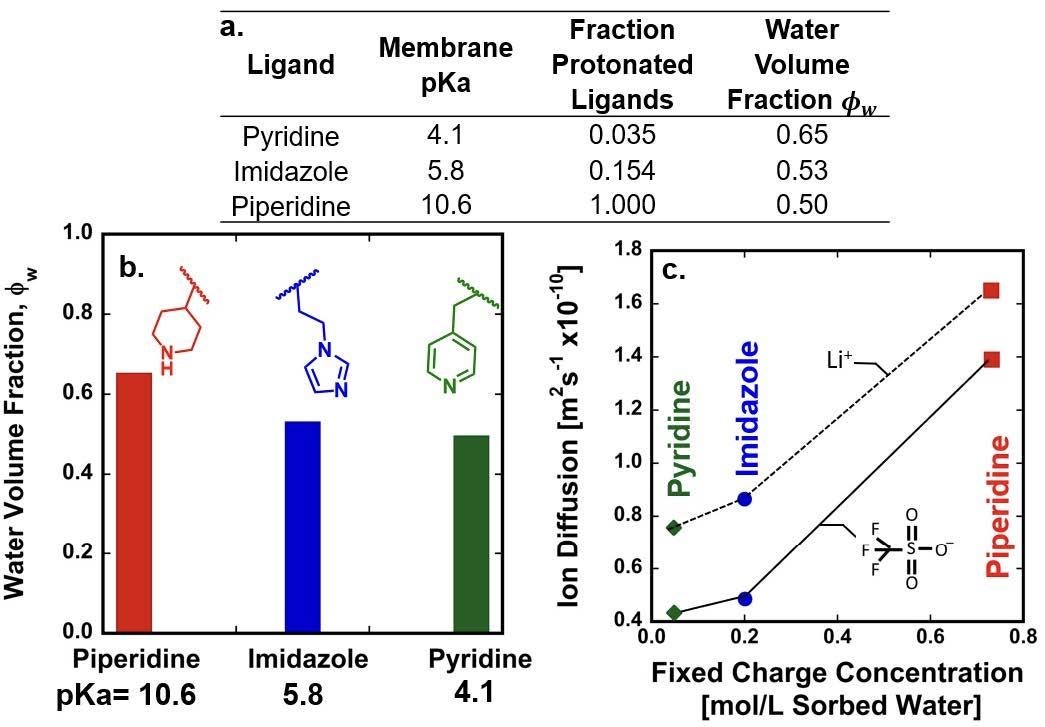
Fig. 3.5. a. Effect of ligand protonation on water content and effective membrane charge density, b. water content is a function of fraction of protonated ligands, c. Ion diffusion is controlled primarily by water content in PEGDA/PEGMEA/acrylate membranes functionalized with ligands of varying Lewis basicity (Fig. 3.3A).

