We will use the unique toolset established in our earlier M-WET work to probe interfacial water properties on the molecular scale necessary to understand and ultimately design surfaces to program surface water.
What is the precise nature of the hydration layer or “barrier” that has been assumed to impact membrane properties? How can this hydration layer be tuned via surface chemistry? We will use the unique toolset established in our earlier M-WET work to probe interfacial water properties on the molecular scale necessary to understand and ultimately design surfaces to program surface water. It is critical to explore and understand the breadth of functionalities necessary to create programmable surface interactions, to ultimately design membranes that are both selective and robust. Using our materials platform capable of producing multifunctionality (Segalman/Hawker), we will experimentally and computationally quantify the role of chemical and topological surface heterogeneity on hydration water dynamics, structure, and thermodynamics. In particular, we will test the idea that a strongly hydrogen bonded hydration water network (i.e., a highly thermodynamically stable hydration layer) is key to antifouling, by comparing hydrophilic surfaces bearing hydrogen bond donors to hydrophilic surfaces without. Similarly, we will probe the role of charges and zwitterions on hydration and water dynamics. These functionalities will first be displayed on water soluble polymers bearing spin probes to understand their influence on local water dynamics. Next, these functionalities will be displayed on micelles where the hydrophobic core forces the display of the surface groups on molecularly flat surfaces at the nanometer scale. Finally, the same functionalities will be displayed in a hydrogel, formatted as a smooth membrane-like surface and as 100 micron particles suspended in water within a capillary suitable for NMR and X-ray characterization.
With these model surfaces, we will leverage the unique suite of tools established in M-WET to address the critical question of how surface chemistry and topology impact measured structural, thermodynamic, and dynamic properties of the membrane hydration layer. Overhauser Dynamic Nuclear Polarization (ODNP) (developed by Dr. Song-I Han) will probe hydration dynamics near surfaces (appropriately spin-labelled) in both suspended functionalized hydrogel particles and micelles. Specifically, we anticipate that functional groups including fixed charges and hydrogen bond donors will alter the dynamics of the surrounding water. Measured ODNP water diffusivities will then be directly compared to Shell’s molecular dynamics (MD) simulations that quantify water diffusivities as well as their relationships to water structure (e.g., tetrahedrality, H-bonding) and thermodynamics (e.g., solvation energies/entropies, activity) (Fig. 2.3). Electronic structure and quantum chemical calculations (Henkelman) will capture the role of ions and charged surface interactions with water and will assist in the parameterization of MD force fields. Measured water dynamics will be complemented by depth-dependent chemical composition and water content in analogous thin films via Ambient Pressure X-ray Spectroscopy (APXPS) (Crumlin). Furthermore, Su will use surface-sensitive near-edge X-ray absorption fine structure (NEXAFS) spectroscopy to decipher functional groups and polymer blocks to quantify surface composition in the top few nanometers of a polymer film. GAP A will leverage powerful characterization methods developed within the IF to further probe surface morphology including nanoscale imaging and scattering methods. By systematically linking surface composition to hydration water response, we will discover design rules for programming hydration water properties and hence modulating solute affinity.
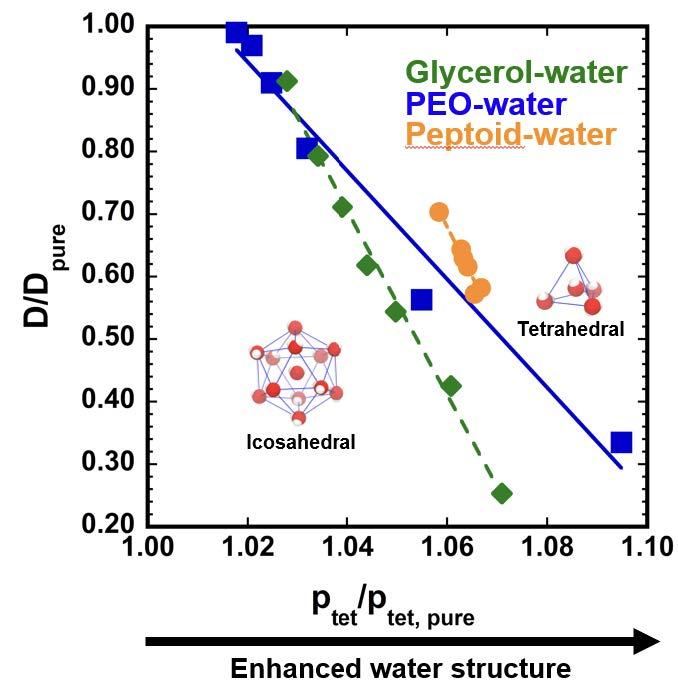
Fig. 2.3. Our prior M-WET work used molecular simulations to establish remarkably strong and persistent relationships between the response of water properties to surfaces and solutes. Here, we show that hydration water dynamics are tightly connected to hydration water tetrahedrality across a range of systems: glycerol-water and PEO-water solutions at different compositions, and peptoids of varied hydrophobic-hydrophilic content and sequence. These results demonstrate that the diffusion coefficient of water is systematically decreased as a function of solute concentration or hydrophobicity, relative to its value in pure water, as the water structure shifts from icosahedral (simple liquid like) to more tetrahedral conformations (water-like). These efforts establish an exciting basis for relating water structural response to functional properties in a predictable manner and for using ODNP measures of local diffusivity and other experimental probes to assess hydration water shifts relevant to solvent affinity.

