Our second focus is understanding how surface chemistry and heterogeneity impact the thermodynamic affinity of solutes to membrane interfaces. We will use the same model surfaces proposed in Project 1 to experimentally and computationally characterize the affinity of MFP solutes to them, establishing a rational framework for engineering solute affinity and repulsion at membrane interfaces.
Our second focus is understanding how surface chemistry and heterogeneity impact the thermodynamic affinity of solutes to membrane interfaces. We will use the same model surfaces proposed in Project 1 to experimentally and computationally characterize the affinity of MFP solutes to them, establishing a rational framework for engineering solute affinity and repulsion at membrane interfaces. We hypothesize that the hydration water response to a surface (e.g., hydration water structure, dynamics) offers key signatures of solute affinity. Thus, we will couple the hydration water insights from Project 1 to solute affinities for a range of MFP solutes, focusing in particular on several important solute families: (1) aromatics (benzene, toluene, phenol, benzonitrile, xylenes, naphthalene, dioxane) that represent contaminant chemistries of concern and that allow us to systematically modulate hydrophobic interactions, (2) linear aliphatics and alcohols that appear in oil foulants and can probe chain length variations, (3) short polypeptides as protein mimics with varied hydrophilic phobic composition, (4) borates and arsenous acid that form molecularly small, neutral species that are difficult to separate, and (5) silicates that aggregate proteins and NOM on membrane surfaces with inter-solute interactions that apparently increase fouling beyond the sum of the two foulants.
We will characterize interfacial solute adsorption as a function of surface chemistries in functionalized hydrogels using a unique workflow: APXPS measurements (Crumlin) will measure precise, depth-dependent concentrations of solutes at surfaces with varying levels of hydration (Fig. 2.4). Su will use NEXAFS spectroscopy at different detection modes with varying surface sensitivity to unveil solute and polymer composition as a function of depth, while Katz will characterize solute affinity (adsorption) in the bulk membrane interior for a variety of background water qualities ranging from simple single solute systems of varying ionic strength to multi-solute systems. This work will be complemented by resonant soft x-ray reflectivity, a unique capability at ALS, which leverages the same energy-resolved chemical sensitivity of NEXAFS spectroscopy and can quantify the depth-resolved composition of specific solutes in a polymer film with nm resolution. Simultaneously, ODNP measurements by Han, both at the surface of an analogous hydrogel particle and in the interior, will report on changes in hydration shell dynamics in response to solute adsorption. Simulations by Shell will simultaneously compute the spatially-dependent solute excess chemical potential (Fig. 2.4a)and activity coefficient, from bulk water, to the membrane interface, and then in the membrane interior. The key observables to compare are the surface partition coefficient K and concentration profile c(z). Shell’s computational inverse design methods combining simulations, machine learning, and optimization algorithms will be used to identify membrane surface chemical compositions or peptoid sequences that minimize surface affinity of representative small foulants.
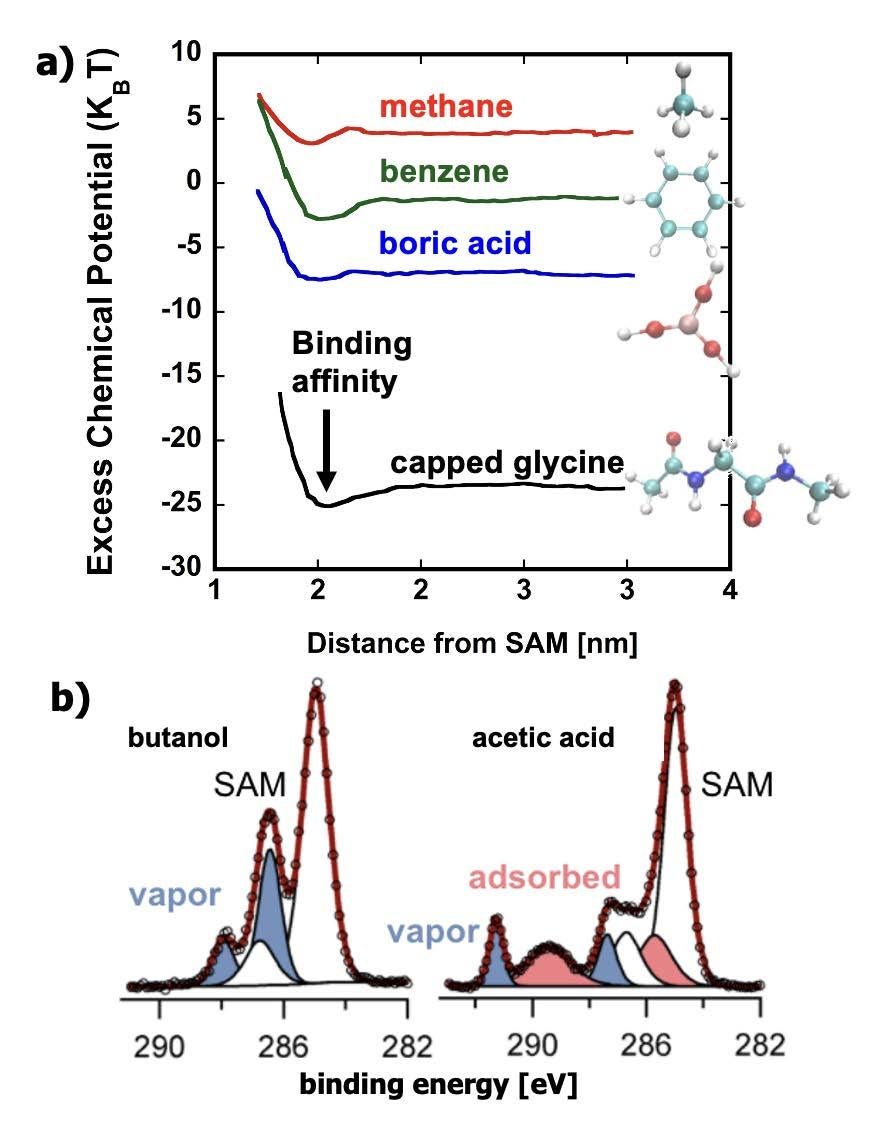
Fig. 2.4. (a) Simulations measure affinity (excess chemical potential / activity) of a range of MFP solutes to model SAM surfaces. (b) APXPS experimentally quantifies adsorption of solutes (butanol, acetic acid) to SAM surfaces.

