Ions are especially complex solutes due to their strongly bound hydration shell, ion pairing, and long-range electrostatic interactions. Therefore, Project 3 in GAP A focuses on a better understanding of the impact of aqueous ion interactions (ion hydration, ion pairing) and functionalized membrane material properties (water density/hydrophobicity, charge density) on ion affinity, dynamics, and membrane electrostatics (e.g., Donnan potential).
We have previously shown the importance of hydration and ion-pairing on membrane selectivity for ionic solutes, such as Na+/Mg2+ and NaCl/Na2SO4 in Nafion. Here, we propose to elucidate the impact of surface chemistry on ion hydration and ion pairing. In situ APXPS can quantify the Donnan potential and ion transport as a function of ionic strength, thus enabling us to refine predictive models of ion selectivity for varying functional groups. X-ray absorption fine structure spectroscopy (NEXAFS/EXAFS) will help reveal local environments around specific ions and nearest neighbor coordination shells.
Utilizing the platform shown in Fig 1.2b and 2.2, we will incorporate functional groups such as sulfonate, carbonate, imidazoles, phosphonates, amines (primary, secondary, and tertiary), and zwitterions into carefully controlled hydrogel films and micelles functionalized with similar functional groups and then evaluate their relative affinity for a range of cations and anions that vary with respect to their size, charge and propensity for hydration and ion-pairing, including alkali metals (Na+, Li+, Cs+), alkaline earth metal ions (Mg2+, Ca2+, Sr2+), transition metals (Cu2+, Zn2+, Cr3+), halides (F-, Cl-, I-), and oxyanions (e.g., nitrate, phosphate, arsenate, sulfate, borate, or chromate). To determine the solute affinity of each ion/functional group (Katz), both concentration and ionic strength will be varied to assess the impact of ionic concentration on hydration and solute affinity in hydrogel films characterized via spectroscopic and macroscopic tools in the IF. Additionally, we will conduct macroscopic studies of material properties (e.g., water and ion permeation and ionic conductivity) to fully characterize them in conjunction with the IF, using crossflow rejection experiments, concentration gradient-driven permeation experiments, electrically driven conductivity measurements, and macroscopic water uptake. Using these composite data together with corresponding molecular dynamics simulations, we will quantify the relative impacts of functionalization and charge density on water dynamics at/near functional groups. This information will address a fundamental question regarding the importance of water dynamics on the competition between ion hydration and ion pairing with respect to solute affinity for the interface bearing the functional group.
We will use in situ APXPS characterization of these materials (Crumlin) to probe the surface chemistry that drives solute (e.g., ion) activity within the membrane and gain atomistic understanding of macroscopic phenomena (Fig. 2.5). We have developed a new flow cell geometry that allows us to change the liquid on the backside of the membrane to enable the direct observation of ion, solute, and solvent transport through the membrane as a function of time. The true power of this capability is that we can now observe transient changes in macroscopic properties (e.g., Donnan potential) for varying molecular level surface chemistry, allowing us to simultaneously explore both thermodynamic and kinetic properties. We aim to further illuminate how electrical potential distributions at membrane/solution interfaces are influenced by size, charge, and chemical interactions among ions. We will expand this approach to study complex ions such as sulfates, phosphates, and nitrates, which can undergo ion pairing, thus influencing their degree of interaction within the membrane. Because these properties play a critical role in influencing the Donnan potential, it will be possible to use results from this matrix of experiments to help reveal sources of nonideal behavior (e.g., activity coefficients) in membranes. These studies will be complemented by ODNP measurements (Han) on micelles bearing fixed charges (as described in Project 1) to understand the role of ions such as alkali metals, alkaline earth metals, and halides as they compete with hydration water to interact with the membrane functional groups (e.g., fixed charge groups). Mixtures of mono- and divalent salts (1:1, 1:2 and 2:1 ratio of anion:cation) with varying propensities for ion pairing will also be studied. MD simulations by Shell will provide a powerful tool for understanding ion surface binding, mechanisms of diffusion, diffusivities, ion-pairing, and ion activity profiles and, importantly, how these properties vary with both the anion and cation species and membrane structure. However, a limitation of our current simulations is that they are based upon fixed-charge, non-polarizable force fields. As M-WET moves forward, we will include Henkelman for his expertise in density functional theory (DFT). While more computationally expensive, DFT is remarkably accurate for the cost and requires no adjustable parameters, allowing us to connect our current strong capabilities in continuum and molecular-scale modeling to atomic-scale interactions governed by quantum mechanics. DFT simulations provide a good description of variable chemical bonding, charge states, and polarizability, which is especially important for ions. DFT will also provide validation for the accuracy of our MD simulations, by comparing predicted interactions between the two for representative small (<1000 atoms) systems. When discrepancies are found, we will use machine learning (ML) to fit corrections to MD force fields using the Henkelman group software package pyamff, providing improved accuracy while maintaining MD’s ability to simulate over long time and length scales. More broadly, the Henkelman group will provide DFT and ML potential capabilities throughout the other GAPs. These collective efforts will establish basic molecular pictures of how membrane surfaces impact ion behaviors, establishing a fundamental thermodynamic and dynamic framework to support efforts in GAP B.
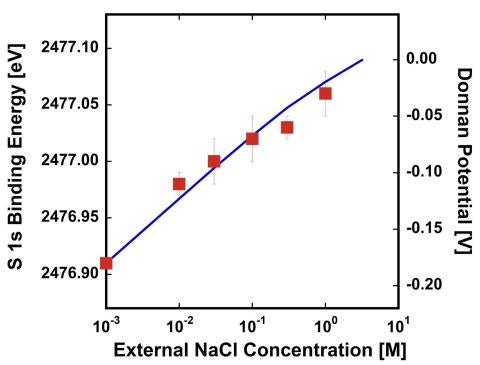
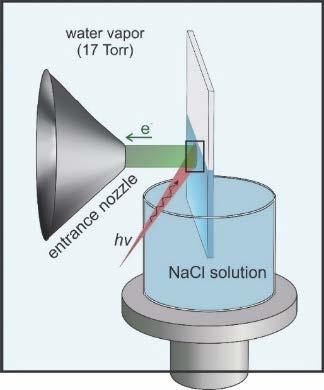
Fig 2.5. APXPS (red data points) provides the first direct measurement of the Donnan Potential at a membrane surface, for NaCl sorbing into a cation exchange membrane (CR-61), achieving quantitative agreement with predictions from the Donnan/Manning model (blue line)

